August 21, 2017
Seema Verma
Administrator
Centers for Medicare & Medicaid Services
Department of Health and Human Services
Attention: CMS-5522-P
P.O. Box 8013 Baltimore, MD 21244-8013
RE: Medicare Program: CY 2018 Updates to the Quality Payment Program
Dear Administrator Verma:
AVAC appreciates the opportunity to offer comments in response to the Medicare Program; Merit-Based Incentive Payment System (MIPS) and Alternative Payment Model (APM) Incentive under the Physician Fee Schedule, and Criteria for Physician-Focused Payment Models. As a stakeholder interested in advancing new physician payment models that encourage access to essential preventive services such as immunization, we are grateful to CMS for its work in this area. AVAC includes more than fifty organizational leaders in health and public health who are committed to addressing barriers to adult immunization. AVAC works towards common legislative and regulatory solutions that will strengthen and enhance access to adult immunization across the health care system. Our mission is informed by scientific and empirical evidence in support of the benefits immunizations provide by improving health, protecting lives against a variety of debilitating and potentially deadly conditions, and saving costs to the healthcare system and to society.
AVAC priorities and objectives are driven by a consensus process with the goal of enabling the range of stakeholders to have a voice in the effort to improve access to and utilization of adult immunizations. A top priority for AVAC is to achieve increased adult immunization rates through federal benchmarks and performance measures that encourage utilization of recommended vaccines.
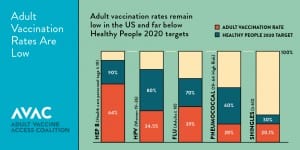
Vaccines protect us from a variety of common diseases that can be serious and even deadly. Every year, more than 50,000 adults die from vaccine preventable diseases and thousands more suffer serious health problems1. Despite Advisory Committee for Immunization Practices (ACIP) recommendations, vaccines are underutilized in the adult population and lag behind Healthy People 2020 goals for the most commonly recommended vaccines (influenza, pneumococcal, Tdap, hepatitis B, herpes zoster, human papillomavirus vaccine (HPV)). Disparities are even greater among at-risk populations, including seniors and people with chronic illness, many of the same vulnerable populations Medicare covers across the country.
The proposed rule seeks to advance payment and policy changes to the Quality Payment Program established by the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA). The law represented a significant step in the transition from a volume-based physician payment model to a system that rewards value.
AVAC values the opportunity to offer our perspective on aspects of the proposed rule that are relevant to the provision of immunizations. Our coalition firmly believes that adult immunization quality measurement is central to ensuring continued focus on this core prevention intervention. AVAC shares your goal of building, strengthening and advancing a new generation of process and outcome measures, as outlined in the CMS Quality Strategy. We are also committed to ensuring this new generation of adult immunization measures bring increased value without adding burden on providers. We look forward to working with you toward improving upon existing adult immunization quality measures.
AVAC believes that adult immunization quality measurement meets the three core strategies underlying the movement toward a truly patient-centered health care delivery system by: 1) Improving the way clinicians are paid to incentivize quality and value of care over simply quantity of services; 2) improving the way care is delivered by providing clinical practice support, data and feedback reports to guide improvement and better decision-making and; 3) making data more available in real-time at the point of contact and enabling the use of certified Electronic Health Record (EHR) technology and other data sources to support care delivery.
Quality
For 2019, the proposed rule calls for the quality performance category to account for 60 percent of a clinician’s composite performance score (CPS), thereby representing a significant portion of their ultimate payment under MIPS. The measures ultimately selected under the quality performance category will have major implications in terms of clinicians’ delivery of care. AVAC appreciates that the 2018 proposed rule maintains important adult immunization measures such as NQF# 0041/110 – Preventive Care and Screening: Influenza Immunization and NQF# 0043/111- Pneumococcal Vaccination Status along with childhood and adolescent immunization measures. The proposed rule also removes cross cutting measures from most of the specialty sets but also seeks comment on ways to incorporate cross-cutting measures into MIPS in the future. With that in mind, AVAC would like to offer the following comments:
Cross-cutting Measures
Cross-cutting measures help focus our efforts on population health improvement. As recognized in Healthy People 2020, prevention of infectious disease through immunization is a key factor in improving the health of our nation. AVAC urges CMS to consider adding the following measures to the list of cross-cutting measures in the future.
➢ Preventive Care and Screening: Influenza Immunization (NQF# 0041/110) — Community/Population Health. Percentage of patients aged 6 months and older seen for a visit between October 1 and March 31 who received an influenza immunization OR who reported previous receipt of an influenza immunization.
➢ Pneumococcal Vaccination Status (NQF# 0043/111) — Community/Population Health. Percentage of patients 65 years of age and older who have ever received a pneumococcal vaccine.
Prior to finalization, the 2017 rule eliminated immunization-related cross-cutting measures for influenza and pneumonia. AVAC believes that all Medicare providers, regardless of whether their scope of practice is focused on primary care or specialty care, should be incentivized to offer immunization services while providing care to patients. The influenza vaccine presents an annual opportunity for a beneficiaries’ main provider, which in the case of chronically ill patient, could be a specialist such as an endocrinologist, a cardiologist or another type of clinician, to review their immunization status and ensure they have access to ACIP-recommended vaccines.
Screening should be done by primary care, as well as specialty providers to ensure that everyone is counseled and has the opportunity to receive the appropriate immunizations, based on their age and health status. Published literature indicates that integrating immunization screening and additional providers offering these critical preventive services will result in greater opportunities for immunization.3. The National Vaccine Advisory Committee’s (NVAC) Adult Immunization Standards call for all providers caring for adult patients to assess, recommend, vaccinate or refer, and document vaccinations.
Advancing Care Information Performance Category (p. 30015)
One quarter (25 percent) of the MIPS final score is based on performance in the advancing care information (ACI) category, which consists of a base score, performance score and potential bonus points for reporting certain measures and activities. The FY18 proposed rule modifies the reporting standard for meeting the Immunization Registry Reporting Measure requirement to accommodate eligible clinicians in parts of the country where immunization registries are not available. While we do not oppose this modification, we believe it is vitally important that the Centers for Medicare and Medicaid Services (CMS) work to support expanded access and reporting to immunization registries across the country. According to the Centers for Disease Control and Prevention (CDC), Immunization Information Systems (IIS), or immunization registries, currently operate in all 50 states, 5 cities, the District of Columbia (D.C.) and 8 territories. While every state in the US presently operates an immunization registry, not all systems are equal, or can connect with all providers in a community. Limited resources and staffing as well as legal and policy barriers hinder the ability of all eligible clinicians in a community to report data to their state or local immunization registry. AVAC urges CMS to work with CDC and its IIS grantees to drive a higher level of interoperability and address legal and policy barriers that prevent Medicare clinicians from reporting data to immunization registries as required under Meaningful Use as well as the Advancing Care Information Performance category. The goal should be for Immunization Registry Reporting to eventually become a required reporting measure under MIPS.
Although immunizations are often administered in a clinical setting, a patient’s lifetime immunization record will span decades, and the consolidation of records as individuals move among health care providers is a unique public health function. It is this consolidated record that drives the accurate forecast of immunizations due, and past due, at the point of care. For this reason, seamless multidirectional interoperability between certified electronic health record technology (CEHRT) and public health in general, and IIS is essential to ensure the provision of appropriate clinical services, and a precursor for accurate measurement of quality care. It is also imperative that all immunization providers, including pharmacists, are able to exchange of relevant clinical information under Certified Electronic Health Record Technology (CEHRT), to ensure CMS and eligible clinicians are able to maximize the benefits of coordinated, team-based care.
Additionally, we recommend that the ACI incentivize and encourage the following:
• Sending reminders to patients using certified EHRs;
• Sending educational information to patients using EHRs;
• Implementing clinical decision support (CDS) tools to identify patients requiring vaccines;
• EHR generated lists of patients requiring immunizations;
• Use of ePrescribing technology to implement electronic, two-way communication between the vaccine-recommending clinician’s chart and that of the vaccinating provider, accomplishing health information exchange (HIE) and the exchange of and access to data between immunization providers within the immunization neighborhood.
These EHR functionalities will strongly promote immunization and assist busy clinicians in assessing, recommending, providing/referring for, and documenting immunizations –the four call-to-actions in the revised Standards for Adult Immunization Practice. Incorporating these functions will facilitate the implementation of technology that exists today but is not fully utilized—and stands as a barrier to increased adult immunization— including IIS reporting and two-way exchange of data between referring clinicians and vaccinating providers in complementary settings, such as pharmacies, hospitals, and health departments.
Moreover, the proposed rule differentiates the Immunization Registry Reporting Measure between active engagement under Stage 2 and Stage 3 reporting. AVAC appreciates that the proposed rule strongly encourages the reporting of immunization data and continues to advance efforts to provide for multidirectional data exchange. Immunization forecasts and patient histories are important tools that strengthen and enhance the ability of clinicians to educate patients and improve clinical decision making at the point of care. Also, ensuring that all immunization providers, including pharmacists, are able to report administered vaccines through an EHR would help to provide a comprehensive database from which to measure patient immunization status.
Lastly, vaccine management in private and public health care settings is an additional area where greater reporting and interoperability would be of benefit. EHR-IIS interoperability is essential to stronger and more efficient vaccine supply management through providing vaccine ordering, inventory, and accountability functions in clinical care settings, both during routine provision of immunizations and in cases of disease outbreaks.
MIPS APM List Comprehensive ESRD care (p. 30093)
AVAC is pleased that two Alternative Payment Model programs included influenza and pneumococcal immunization measures. Influenza and pneumonia are particularly dangerous for persons who are immunocompromised or who suffer from chronic conditions. Preventive measures such as immunizations can help to prevent costly hospitalizations, serious complications and even early mortality. Effective immunization programs can also help to prevent the transmission of deadly and debilitating infectious conditions among medically fragile populations within a community. The specific measures are listed below for the ESRD Care APM list as well as the Comprehensive Primary Care Plus (CPC+) Model APM list.
➢ Influenza Immunization for the ESRD Population (NQF# 0041/110). Community/Population Health. Percentage of patients aged 6 months and older seen for a visit between October 1 and March 31 who received an influenza immunization OR who reported previous receipt of an influenza immunization.
➢ Pneumococcal Vaccination Status (NQF# 0043/111).
Community/Population Health. Percentage of patients 65 years of age and older who have ever received a pneumococcal vaccine.
MIPS APM List Comprehensive Primary Care Plus (CPC+)
➢ Preventive Care and Screening: Influenza Immunization (NQF# 0041/110). Community/Population Health. Percentage of patients aged 6 months and older seen for a visit between October 1 and March 31 who received an influenza immunization OR who reported previous receipt of an influenza immunization.
➢ Pneumonia Vaccination Status for Older Adults. (NQF#0043/111). Community/Population Health. Percentage of patients 65 years of age and older who have ever received a pneumococcal vaccine.
MIPS Specialty Measure Sets (p. 30271)
The 2018 Quality Payment Program proposed rule added immunization quality measures to several specialty measure sets. AVAC is encouraged to see that the following specialty sets include immunization related process quality measures:
➢ Allergy/Immunology. NQF# 0041 Preventive Care and Screening: Influenza Immunization and NQF# 0043 Pneumonia Vaccination Status for Older Adults
➢ Family Medicine. NQF# 0041 Preventive Care and Screening: Influenza Immunization, NQF# 0043 Pneumonia Vaccination Status for Older Adults and NQF # 1407 Immunizations for Adolescents
➢ Infectious Disease. NQF# 0041 Preventive Care and Screening: Influenza Immunization, NQF# 0043 Pneumonia Vaccination Status for Older Adults and NQF # 1407 Immunizations for Adolescents
➢ Nephrology. NQF# 0041 Preventive Care and Screening: Influenza Immunization, NQF# 0043 Pneumonia Vaccination Status for Older Adults
➢ Obstetrics/Gynecology. NQF# 0041 Preventive Care and Screening: Influenza Immunization.
➢ Otolaryngology. NQF# 0041 Preventive Care and Screening: Influenza Immunization, NQF# 0043 Pneumonia Vaccination Status for Older Adults
➢ Pediatrics. NQF# 0041 Preventive Care and Screening: Influenza Immunization and NQF #0038 Childhood Immunization Status and NQF # 1407 Immunizations for Adolescents
➢ Preventive Medicine. NQF# 0041 Preventive Care and Screening: Influenza Immunization and NQF# 0043 Pneumonia Vaccination Status for Older Adults
➢ Rheumatology. NQF# 0041 Preventive Care and Screening: Influenza Immunization, NQF# 0043 Pneumonia Vaccination Status for Older Adults
AVAC was, however, disappointed that the proposed rule did not include quality measures aimed at patients at greater risk of serious complications from vaccine preventable illness. The ACIP includes age-based, as well as condition-specific recommendations for adult vaccination. For instance, patients living with chronic conditions such as heart disease and diabetes are at a significantly higher risk of complications and death from influenza and pneumonia. The CDC has reported that in 2013 only 21.2% of adults in this group had received a pneumococcal vaccination, and this number has been essentially unchanged for at least a decade.
Additionally, individuals with diabetes are at increased risk for hepatitis B infection. As such, the ACIP recommends hepatitis B vaccination for all patients with diabetes age 607 and under as well as other at-risk patients, such as those living with HIV/AIDS and chronic kidney disease. We strongly encourage CMS to add the following immunization quality measures into these specialty measure sets:
➢ Internal Medicine. NQF# 0041 Preventive Care and Screening: Influenza Immunization and NQF# 0043 Pneumonia Vaccination Status for Older Adults.
➢ Endocrinology. NQF# 0041 Preventive Care and Screening: Influenza Immunization and NQF# 0043 Pneumonia Vaccination Status for Older Adults.
➢ Cardiology. NQF# 0041 Preventive Care and Screening: Influenza Immunization and NQF# 0043 Pneumonia Vaccination Status for Older Adults.
➢ Hemotology. NQF# 0041 Preventive Care and Screening: Influenza Immunization and NQF# 0043 Pneumonia Vaccination Status for Older Adults.
General Surgery. NQF# 0041 Preventive Care and Screening: Influenza Immunization and NQF# 0043 Pneumonia Vaccination Status for Older Adults.The proposed rule notes that Section 1848(q)(2)(C)(ii) of the Act allows the Secretary to use measures from other CMS payment systems, such as measures for inpatient hospitals, for purposes of the quality and resource use performance categories. AVAC also appreciates that the 2018 proposed rule encourages the submission of potential quality measures regardless of whether such measures were previously published in a proposed rule or endorsed by an entity with a contract under section 1890(a) of the Act. AVAC urges CMS to look broadly across payment systems under its purview and incorporate a broad array of relevant adult immunization quality measures from other clinical settings, such as the Herpes Zoster (Shingles) vaccination process measure being utilized in the home health value-based payment program – Herpes Zoster (Shingles) Vaccination: Has the Patient Ever Received the Shingles Vaccination?
Considerations for Social Risk (p. 30134)
AVAC appreciates that the CMS continues to study and work with stakeholders to develop and utilize measures that appropriately account for risk factors such as socioeconomic and demographic characteristics, ethnicity and individual health status. We would also urge CMS to carefully consider the overall impact of the resource use measure on immunization services. We understand CMS plans to continue its review of recommendations from the HHS Office of the Assistant Secretary for Planning and Evaluation (ASPE) on the issue of social risk factors. While disparities in childhood immunization rates by race/ethnicity have largely disappeared, disparities in immunization coverage rates along racial and ethnic lines continue to persist across the range of recommended adult immunizations.
The proposed rule seeks comment on the most appropriate social risk factors for stratifying measure scores and/or potential risk adjustment of a measure. The proposed rule notes that social risk factors include, but are not limited to the following: Dual eligibility/low-income subsidy; race and ethnicity; and geographic area of residence. Annual surveillance reports on vaccination coverage among adult populations stratify vaccination coverage by race/ethnicity. The 2015 Surveillance report found that “racial/ethnic differences in vaccination coverage persisted for all seven vaccines” and “Blacks, Hispanics and Asians had lower vaccination coverage than that of whites for all of the vaccines routinely recommended for adults, with just a few exceptions.”AVAC would urge CMS to consider including race/ethnicity in social risk factors for stratifying measure scores and/or potential risk adjustment of a particular measure.
AVAC would also urge CMS to consider an adjustment for socioeconomic status to ensure that clinicians who care for a disproportionate number of low-income beneficiaries are not inadvertently disadvantaged under the resource use calculation relative to their counterparts. It is important to understand the unique and relatively complex nature of immunization services for clinicians. Many providers struggle with storage, inventory, and payment hurdles for vaccines. Managing all of these aspects under a capitated arrangement can actually result in declines in vaccine utilization. It is vitally important that unique cost and management challenges are accounted for in quality measure reporting and do not create a disincentive for providers serving low-income/minority populations to offer important preventive services such as recommended immunizations. Standardizing the offering of vaccines has been shown to reduce differences in vaccination rates.
Immunizations have demonstrated “effective prevention” in reducing rates of morbidity and mortality from a growing number of preventable conditions and improving overall health in a cost-efficient manner. Reducing the number of missed immunization opportunities is imperative to improving health and reducing the burden of vaccine preventable illness among Medicare beneficiaries. AVAC looks forward to working with CMS to ensure that adult immunization quality measures remain an integral component of the Quality Payment Program and are a focus of Alternative Payment Models (APMs) in the coming years as well.
Thank you for this opportunity to offer our perspective on this proposed rule. Please contact the AVAC Coalition Manager at (202) 540-1070 or info@adultvaccinesnow.org if you wish to further discuss our comments. To learn more about the work of AVAC visit www.adultvaccinesnow.org.
Sincerely,
Alliance for Aging Research
American Immunization Registry Association (AIRA)
Asian Pacific Islander American Health Forum
BIO
Dynavax
Every Child By Two (ECBT)
GSK
Infectious Diseases Society of America (IDSA)
Immunization Action Coalition
Immunization Coalition of Washington DC
National Association of County and City Health Officials (NACCHO)
Novavax
Sanofi
Sequirus
Takeda Vaccines, Inc.
The Gerontological Society of America
Trust for America’s Health